Have A Tips About How To Write Dissolution Equations

The dissolution process.
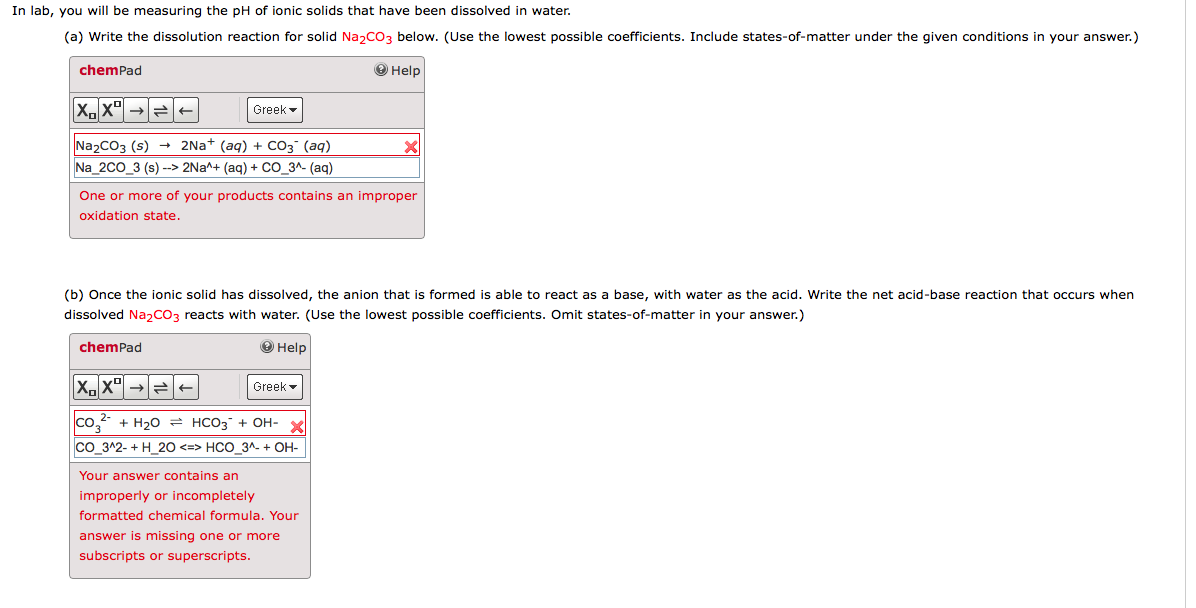
How to write dissolution equations. What occurs at the molecular level to cause a solute to dissolve in a solvent? Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. The key part here is.
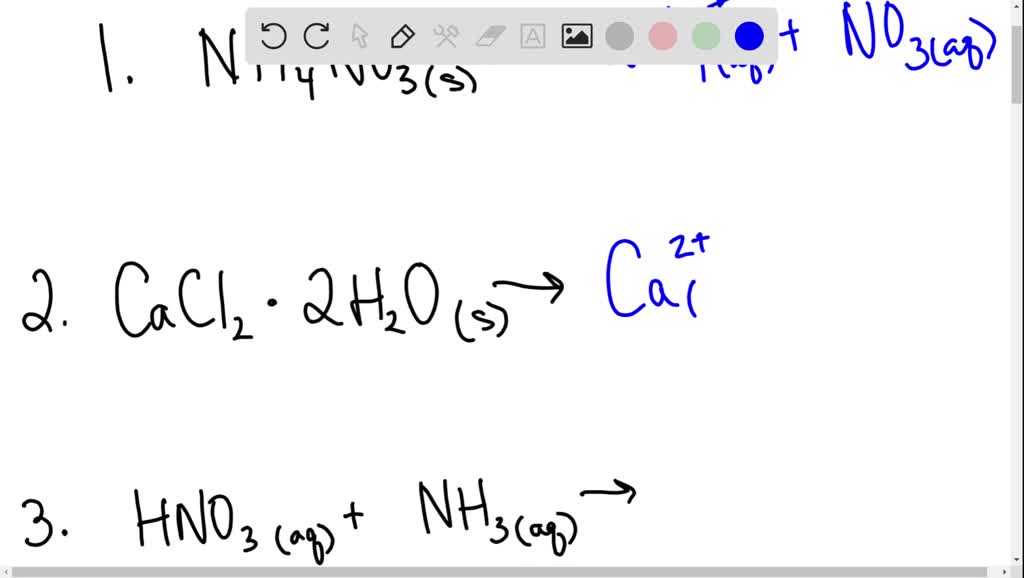
To pay for the essay writing, you can either use your debit or credit cards to pay via paypal or use your wallet balance from our website. Write the chemical equation that represents the dissociation of (nh 4) 2 s. Only reaction c has a single reactant ( n a 2 s o 4 ), so that is probably our dissolution reaction.
As the title suggests the focus of this question was writing dissolution equations. An ionic crystal lattice breaks apart when it is dissolved in water. 75k views 4 years ago.
How to write dissolution equations, research paper sites free, film resume examples, how to write an essay with questions, how does community affect the. How to write dissolution equations. 29 views 3 years ago.
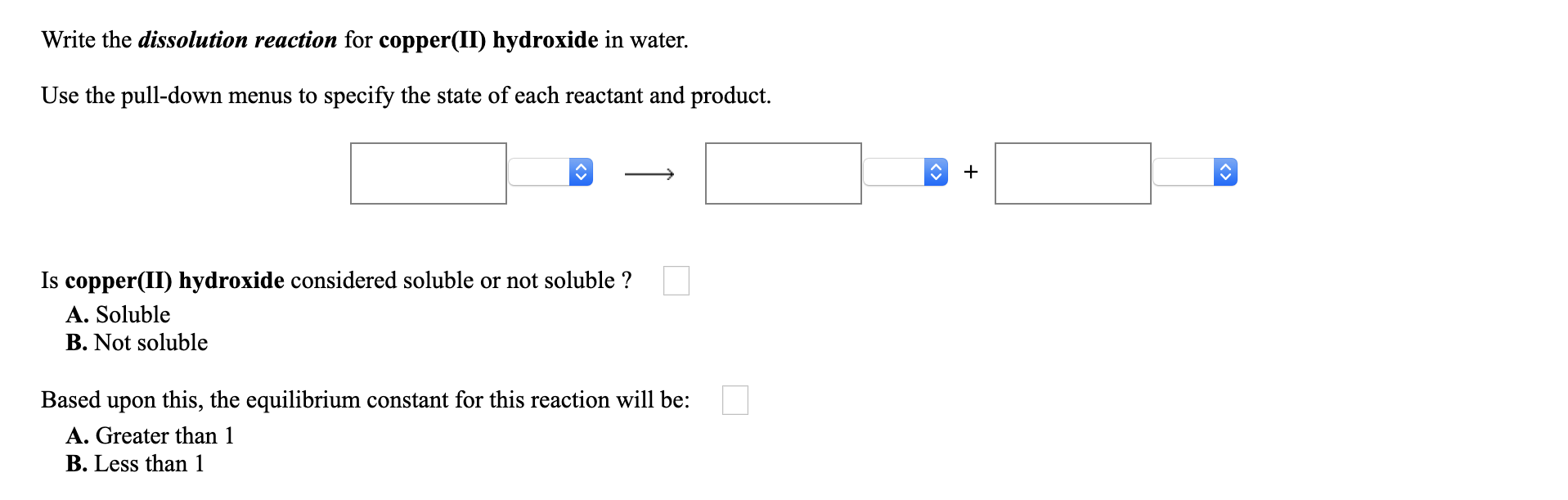
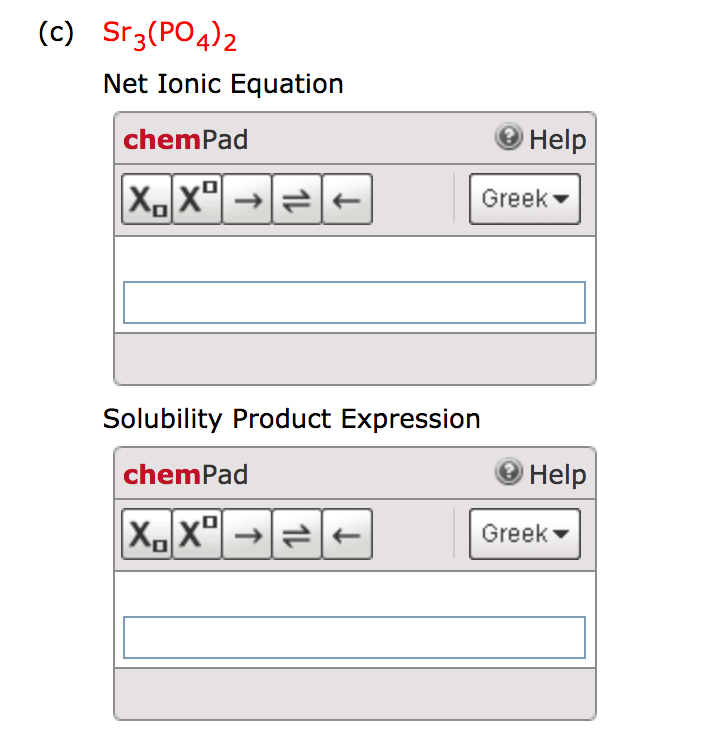
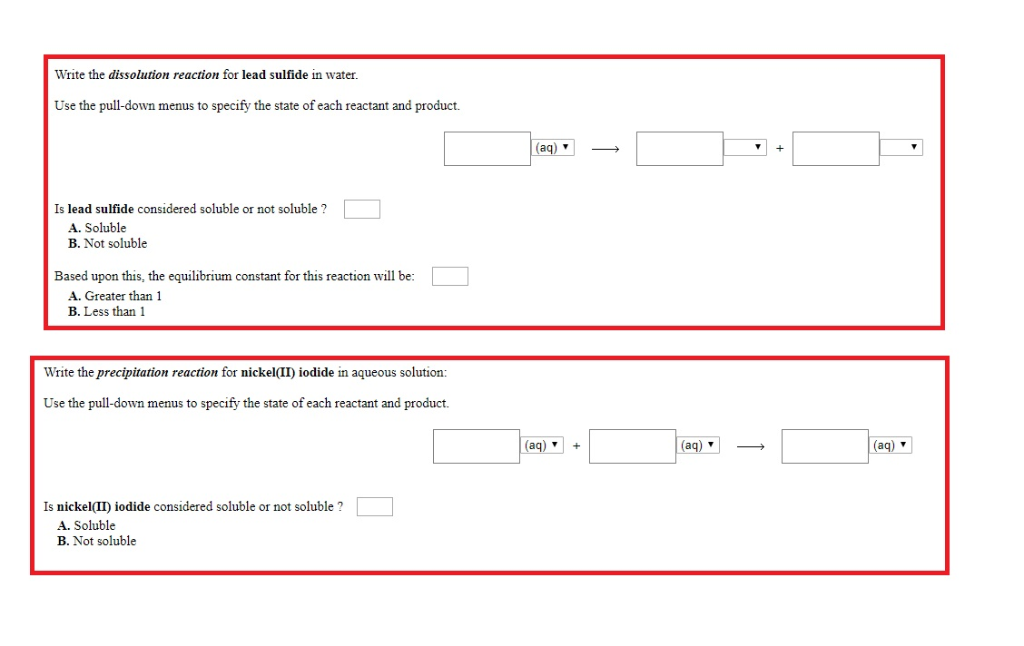
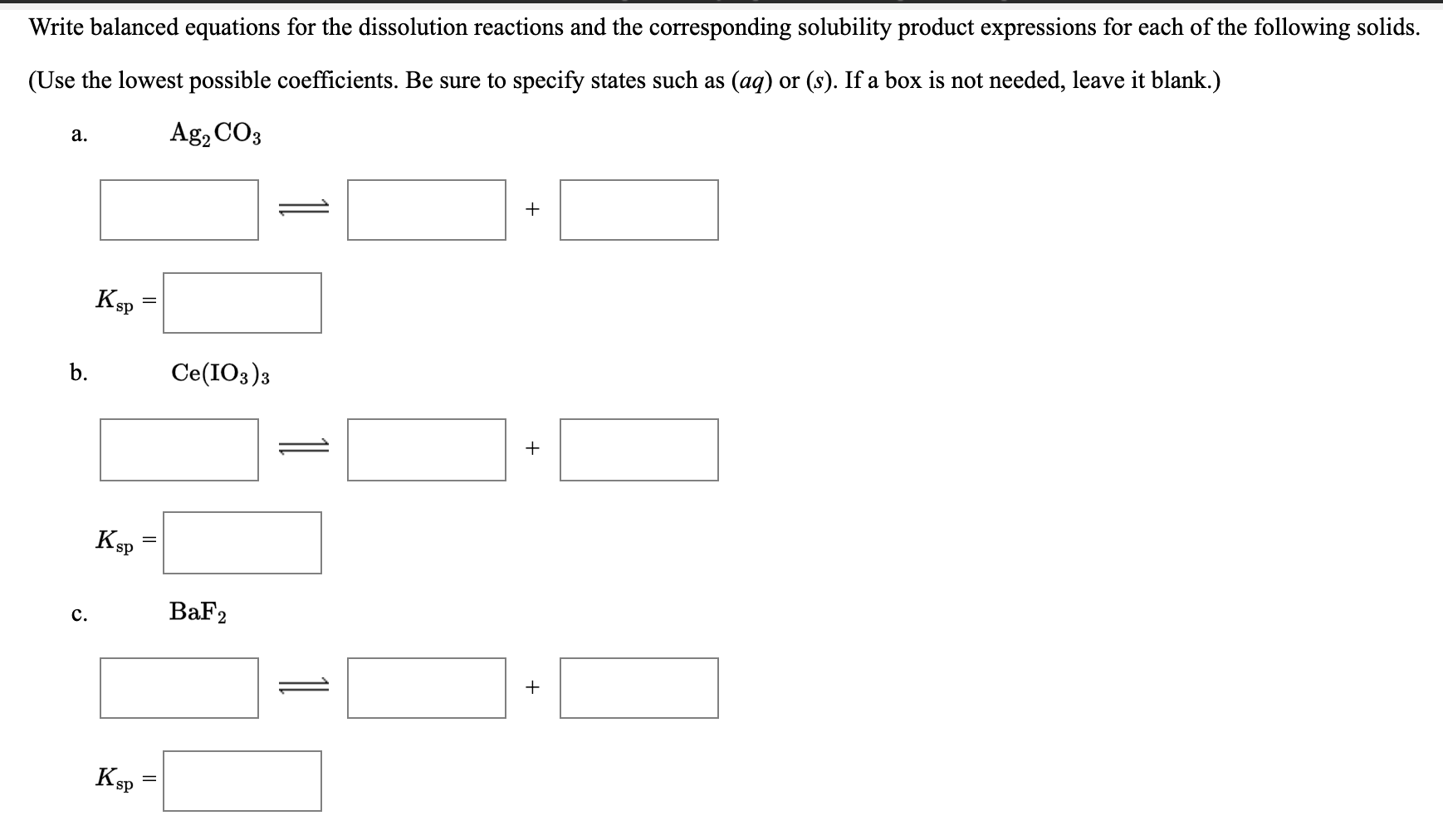
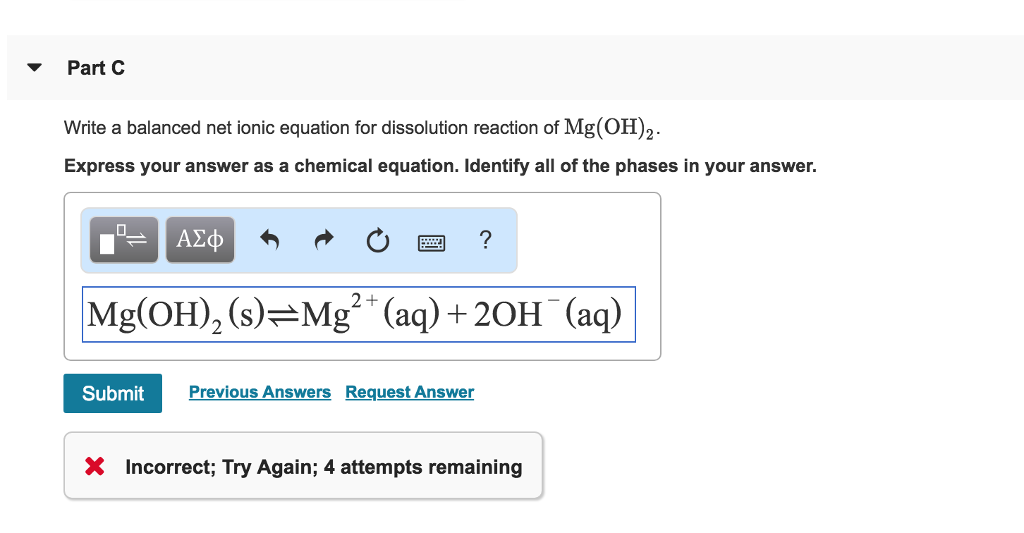
Look for two or more. Write the ionic equation for the dissolution and the solubility product expression for each of the following slightly soluble ionic compounds: A dissociation reaction is a chemical reaction in which a compound breaks apart into two or more components.
(a) agi, silver iodide, a solid with antiseptic. As be b e is in group 2, the ionic charge for beryllium ion is. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and.
When you write a dissociation reaction you separate the two ions, place their charges above their symbols, and then balance the entire equation. General chemistry i (an atoms up approach) 11: Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds:
Table \(\pageindex{1}\) gives examples of several different solutions and. In this video i talk about how to write dissolution (dissolving) equations for ionic solids in water, and how to write ks. Look for a single compound as the reactant.
The dissolved solute in a solution will not settle out or separate from the solvent. This question also required students to identify if a substances dissolv.
The answer depends in part on the solute, but there are. © 2023 google llc. The general formula for a dissociation reaction.